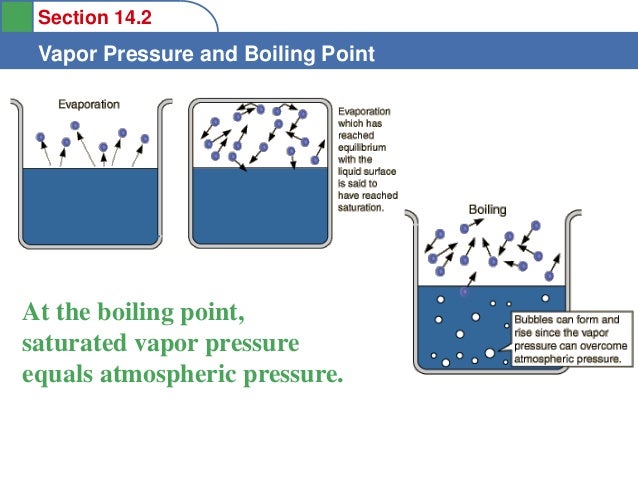
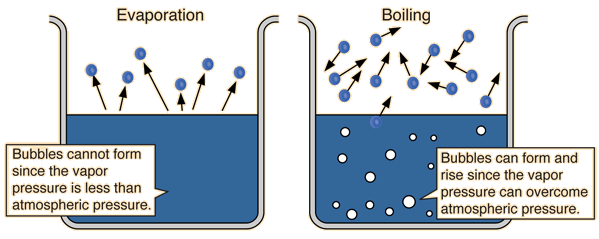
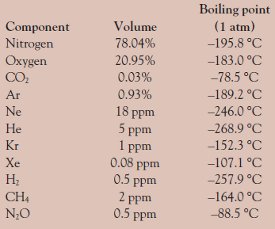
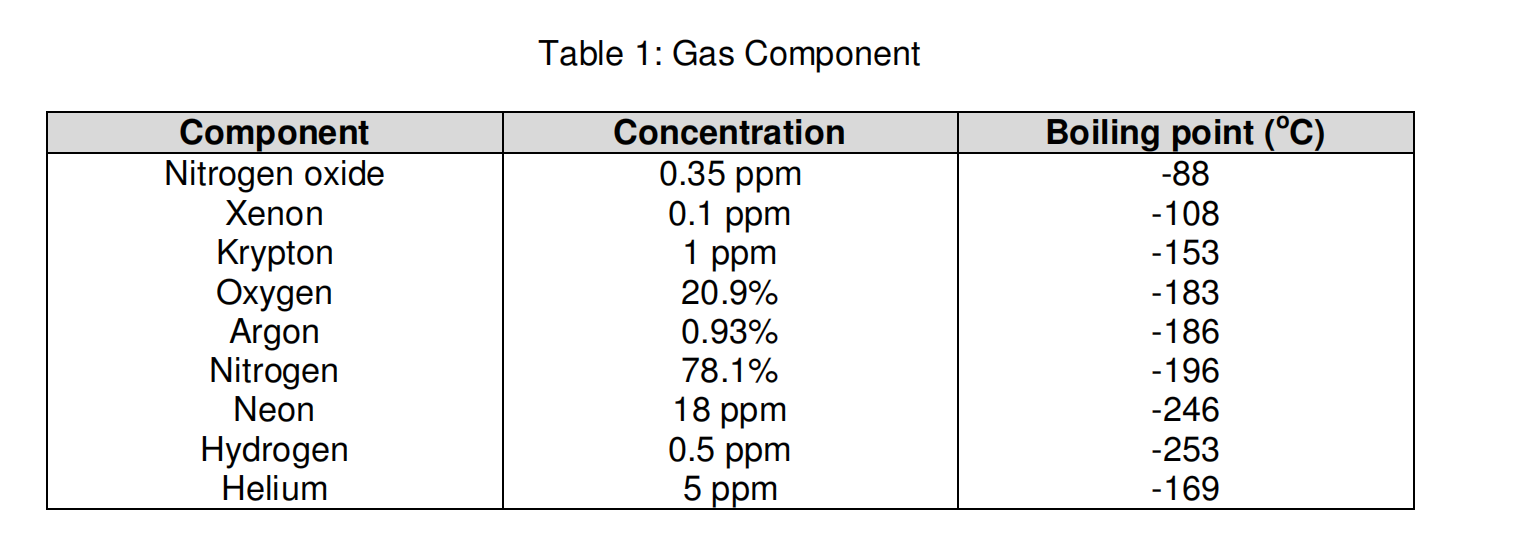
its normal boiling point is -189°C. Oxygen is a gas at room temperature. If the normal melting point of a substance is below room temperature, the substance is a liquid at room temperature. Benzene melts at 6°C and boils at 80°C; it is a liquid at room ...

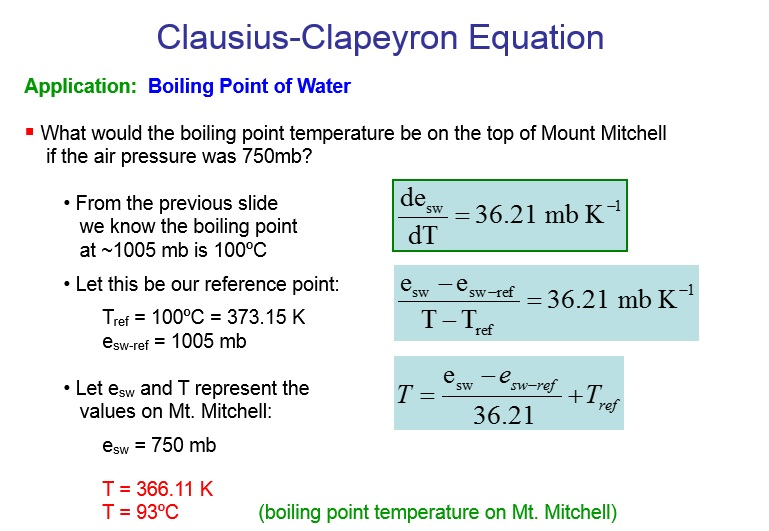
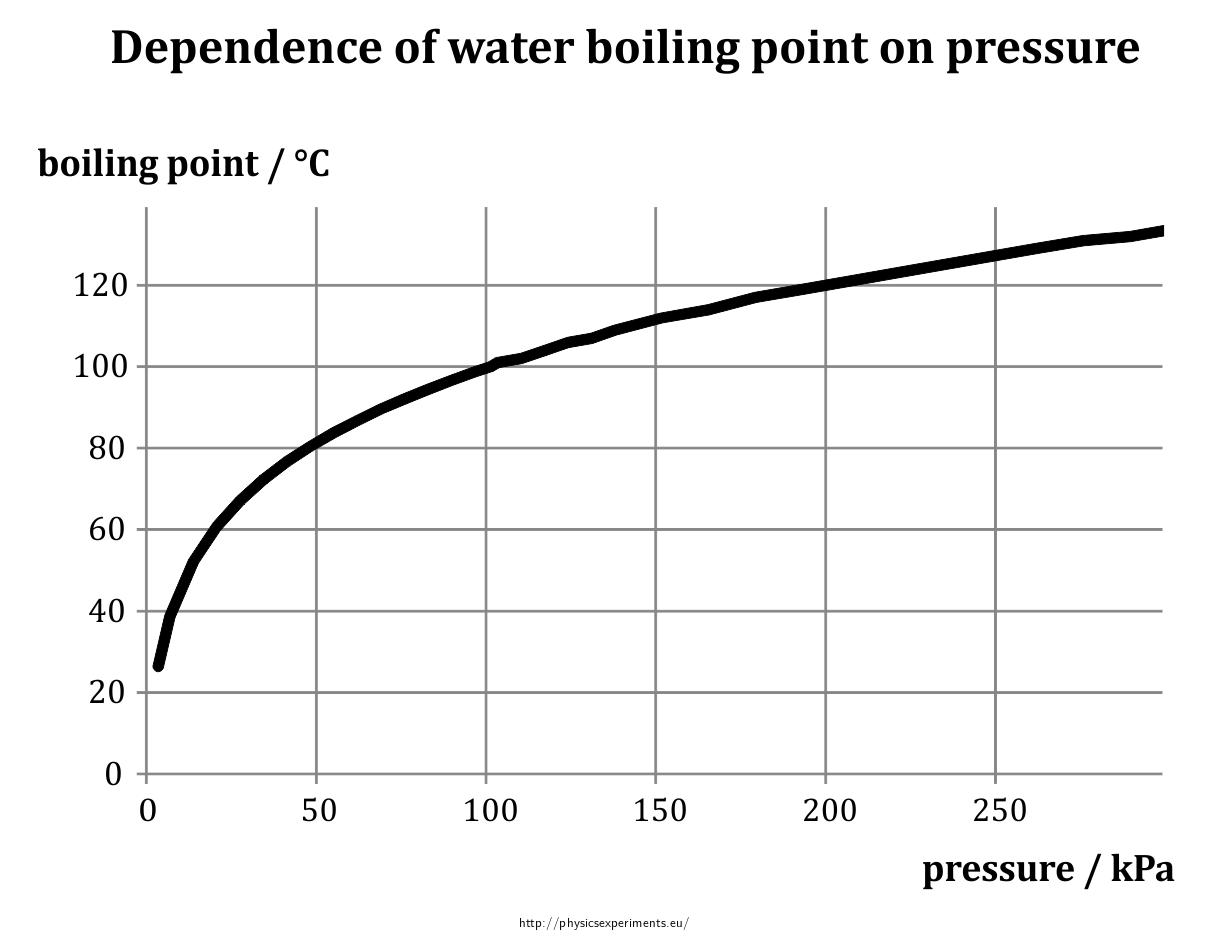
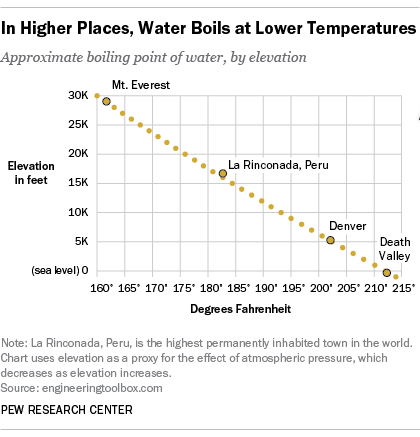
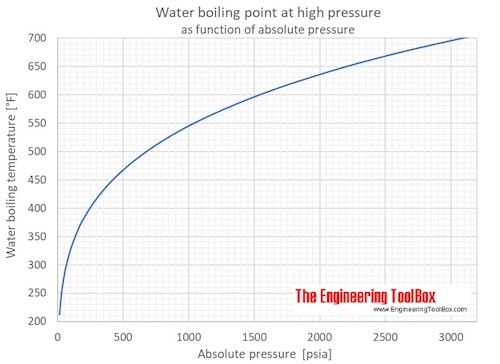
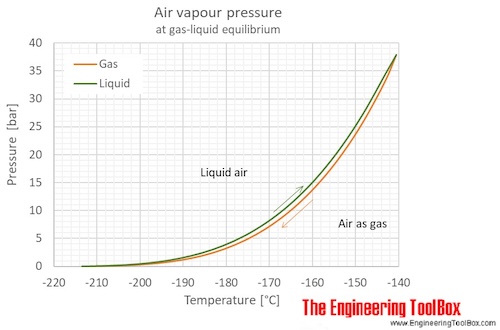
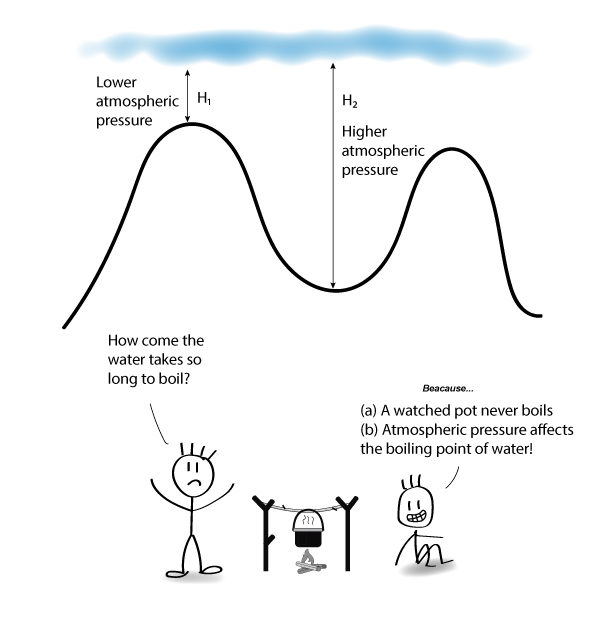
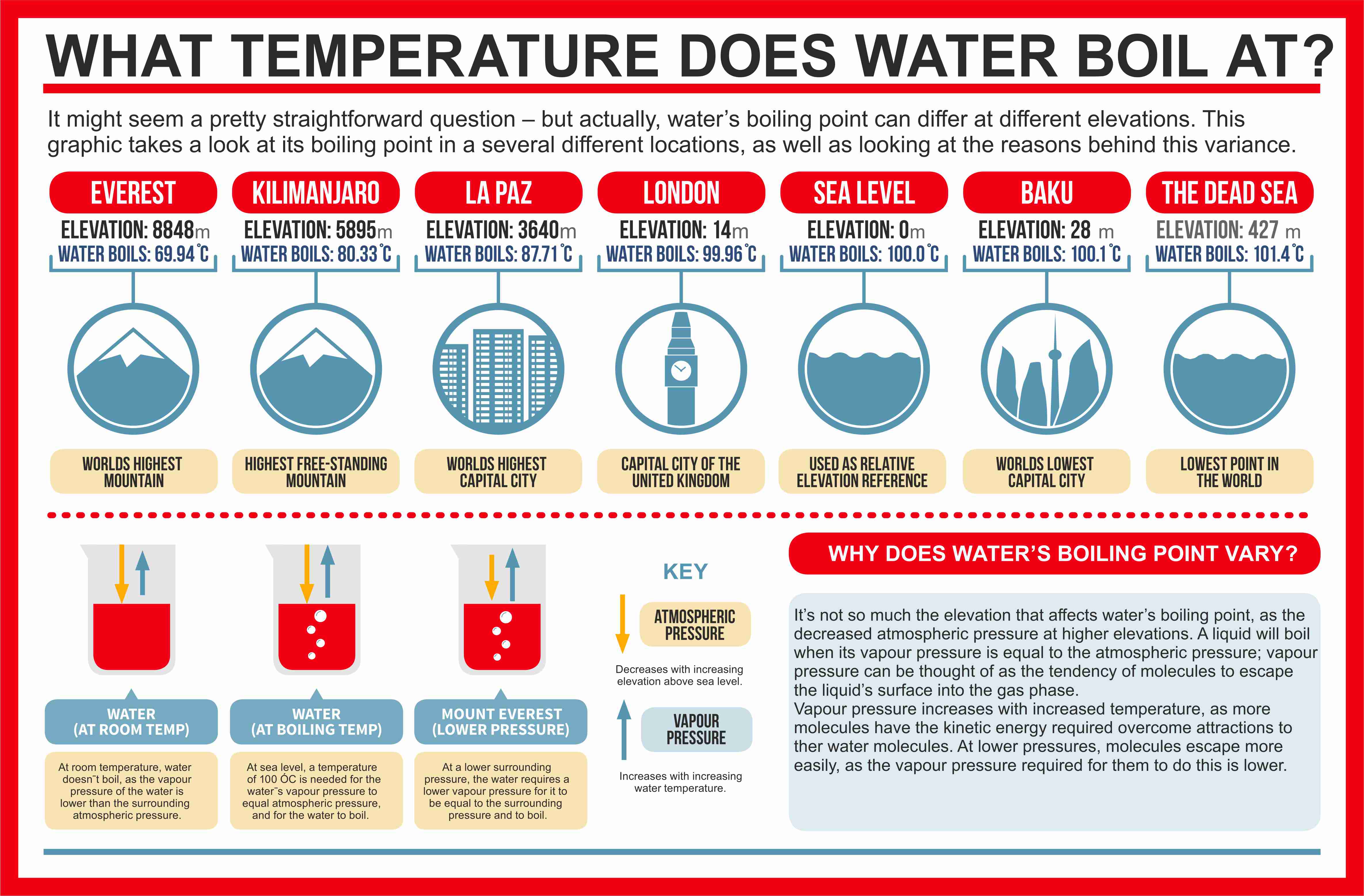
The boiling point of water is100Cat an atmospheric pressure of1atmosphere or100kPa a typical level at sea level At higher altitudes the air pressure decreases and the boiling point increases Study the graph