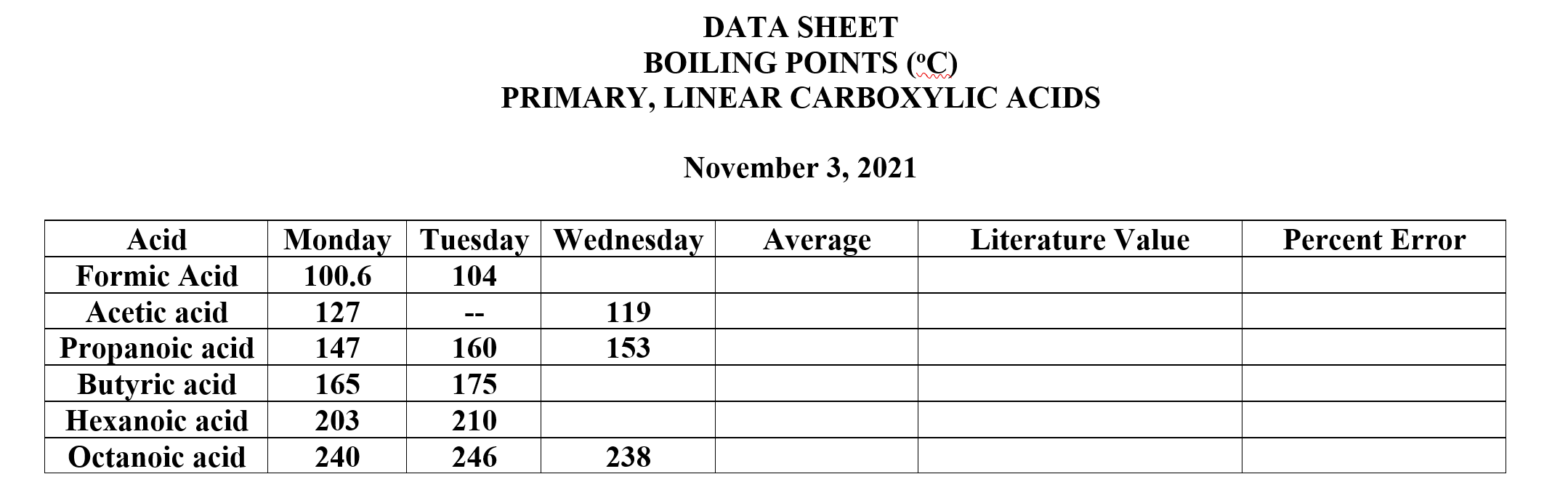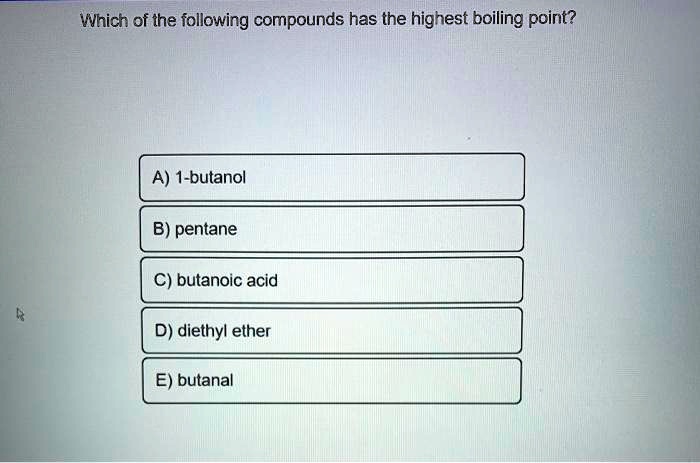
SOLVED: Which of the following compounds has the highest boiling point? A) 1-butanol B) pentane butanoic acid D) diethyl = ether E) butanal

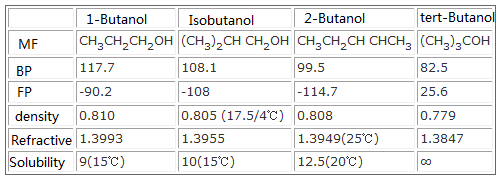
Pentane has a boiling point of 36.1 degrees Celsius while 1-butanol, which has a similar mass, has a boiling point of 117.7 degrees Celsius. Explain this difference, including line-angle structures of each

Arrange the following in increasing order of boiling point: n-butane,n- butanol,n-butylchloride, - YouTube
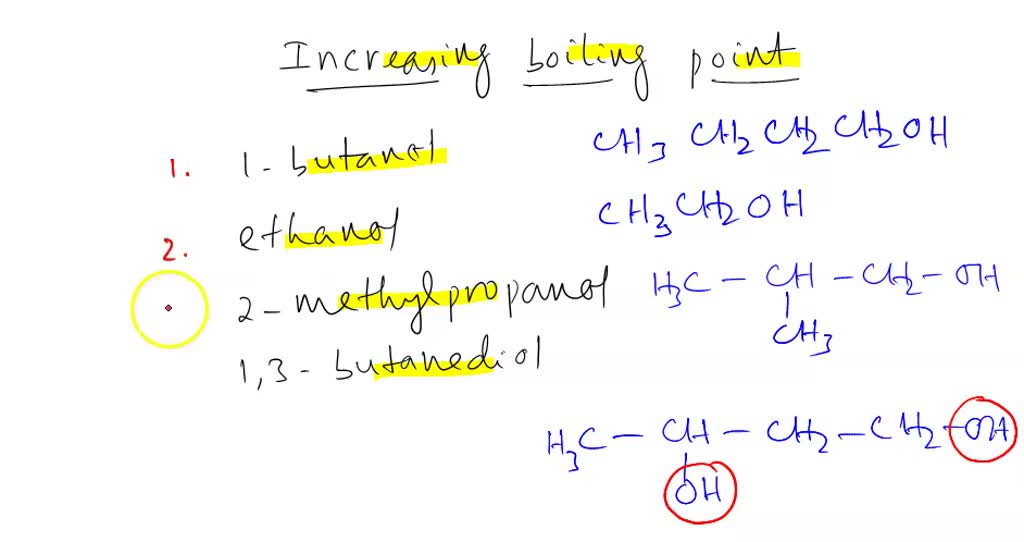
SOLVED: list the following increasing boiling point: 1-butanol, ethanol, 2-methyl-propanol and 1,3-butanediol

why butanol has higher boiling point than butan-2-ol - Chemistry - Alcohols Phenols and Ethers - 13271109 | Meritnation.com

Difference Between 1 Butanol and 2 Butanol | Definition, Chemical Properties, Chemical Structure and Differences
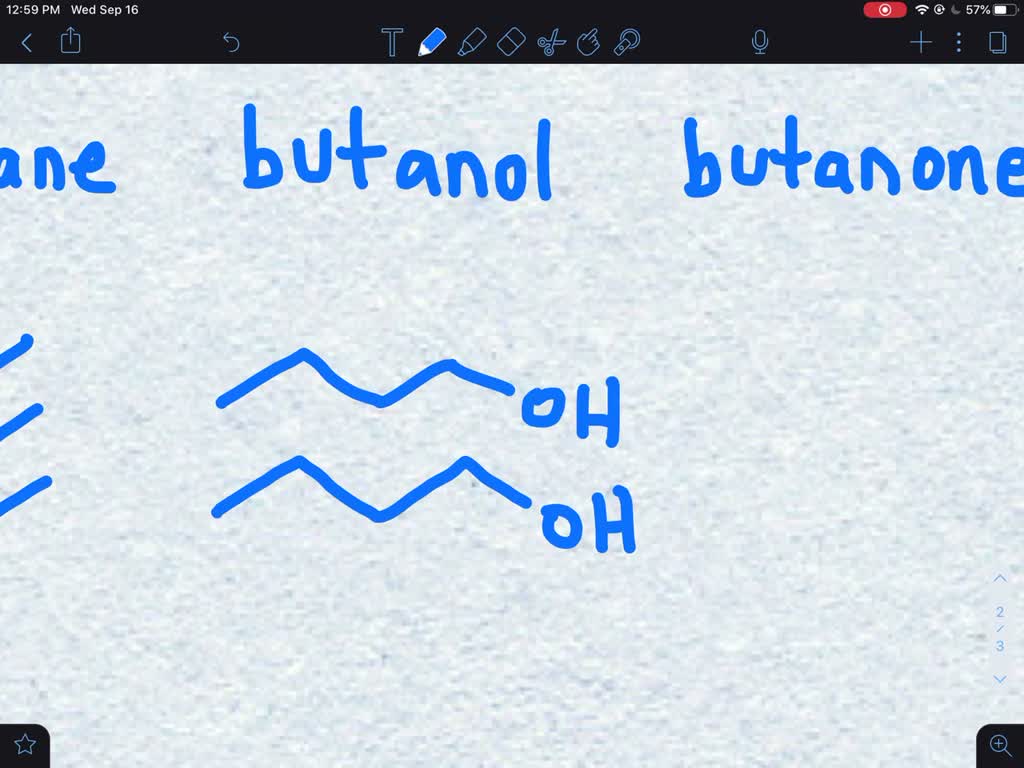
SOLVED:Order the following compounds by increasing boiling point: butane, butanol, butanone (A) Butanol < butane < butanone (B) Butane < butanone < butanol (C) Butanone < butane < butanol (D) Butane < butanol < butanone