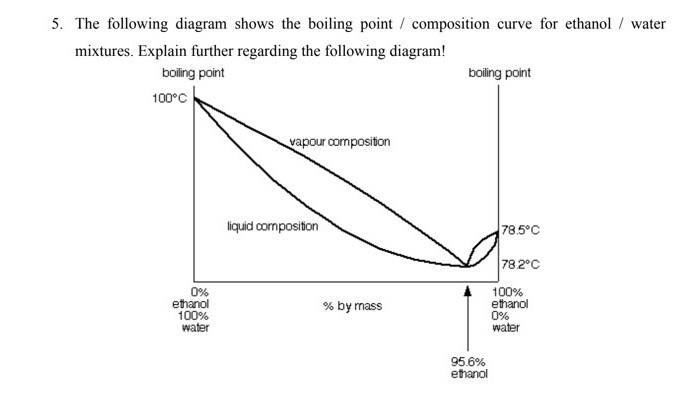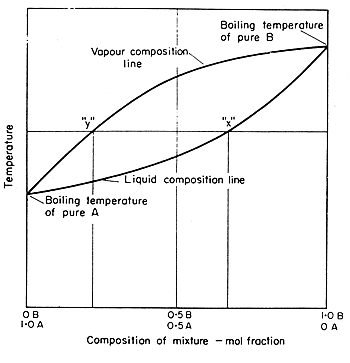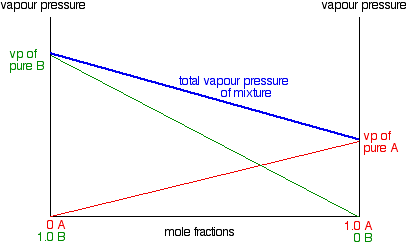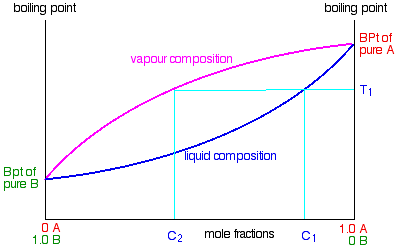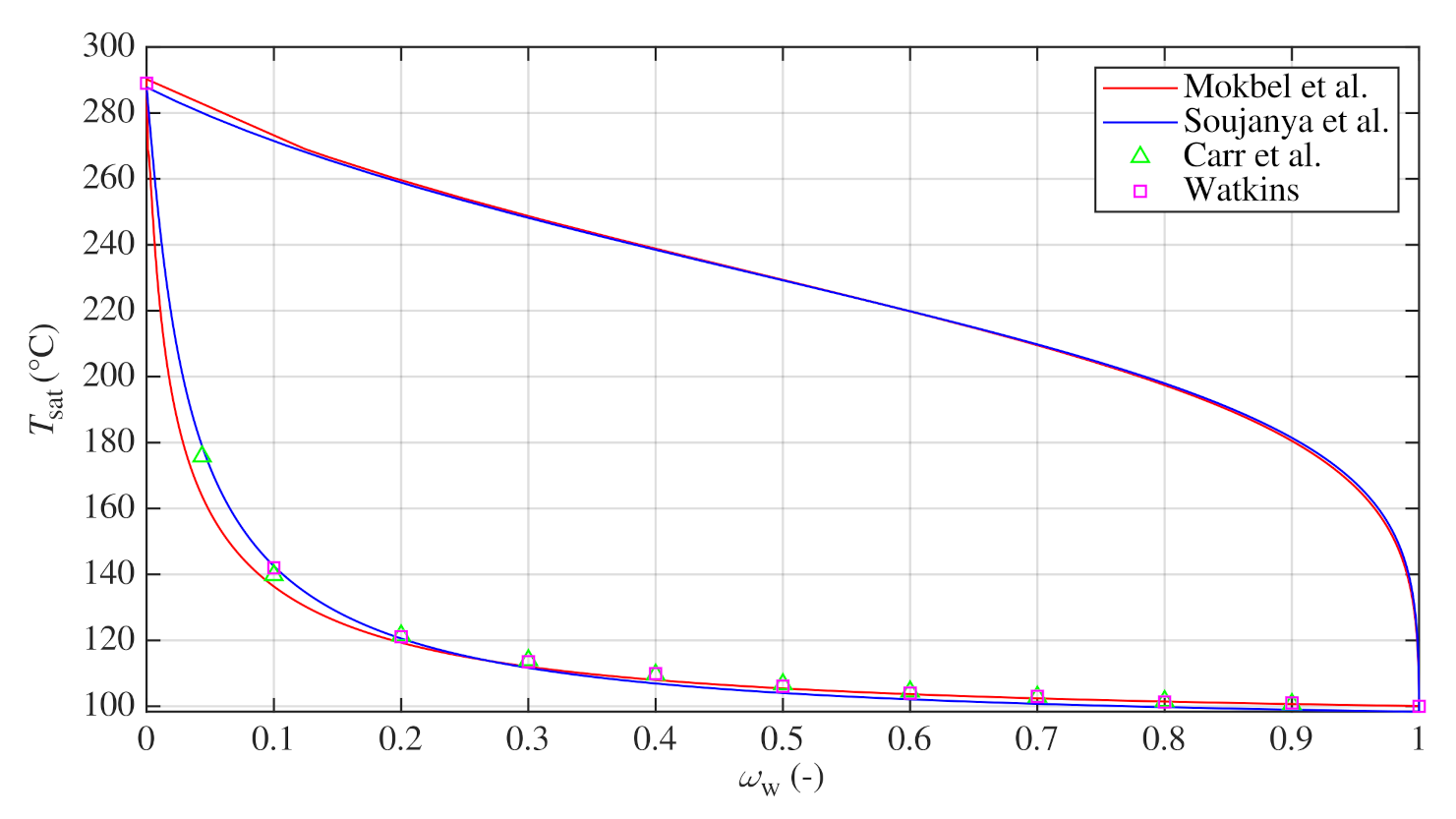
Processes | Free Full-Text | Pool Boiling Heat Transfer Coefficients in Mixtures of Water and Glycerin
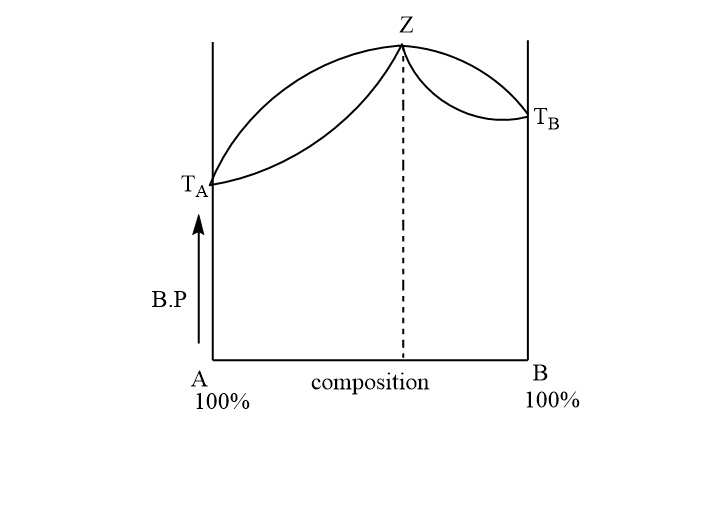
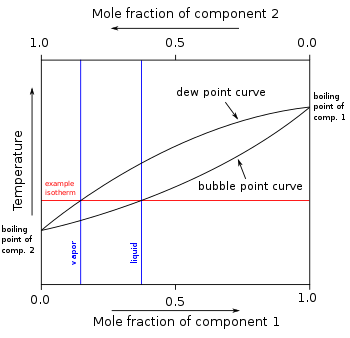
A liquid mixture having composition corresponding to point z in this figure shown is subjected to distillation at constant pressure. Which of the following statements is correct about the process?\n \n \n \
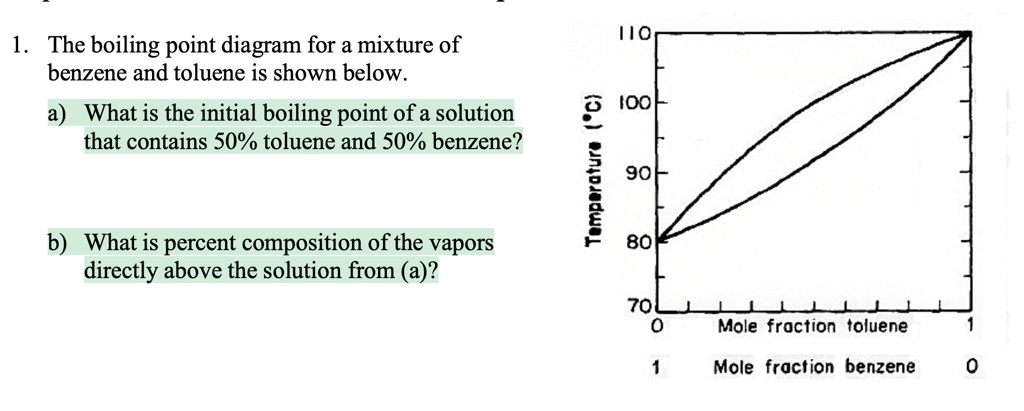
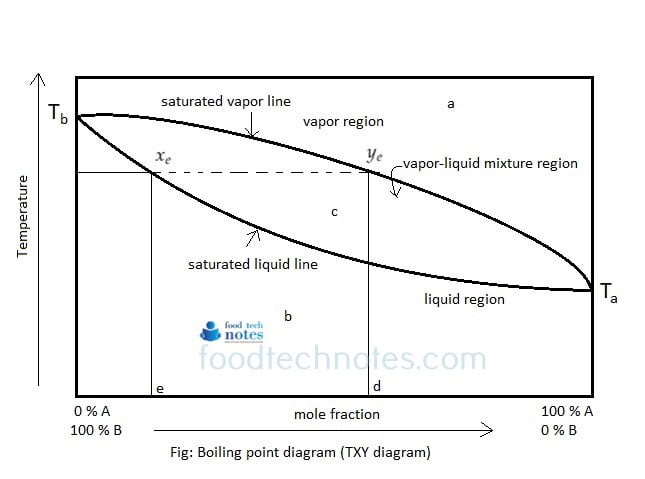
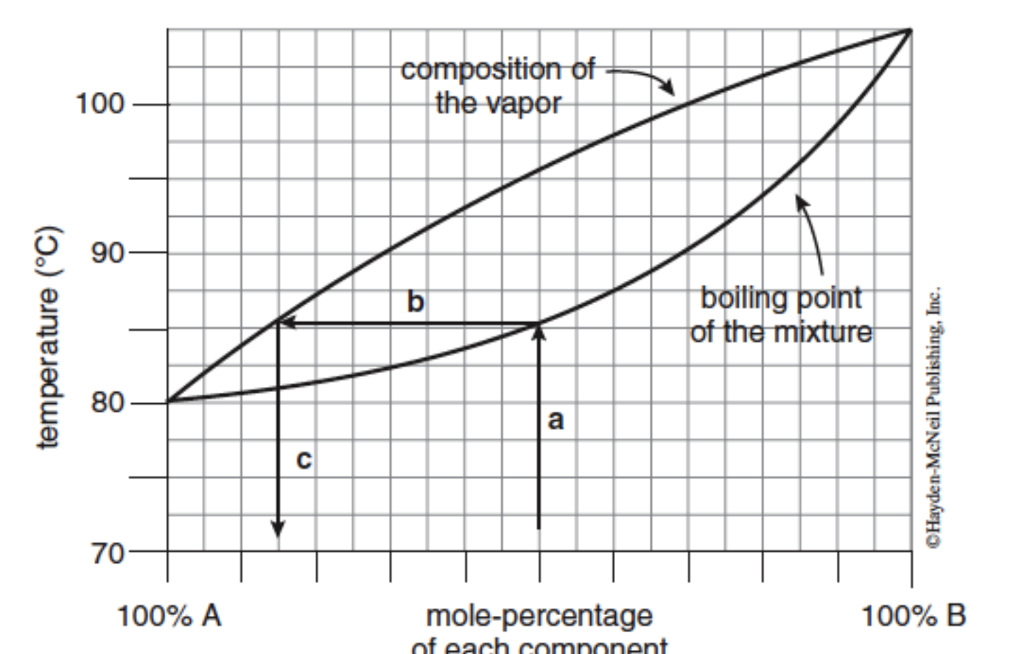
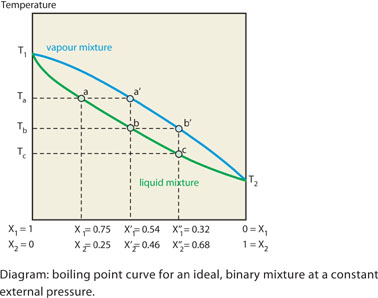
SOLVED: 1. The boiling point diagram for a mixture of benzene and toluene is shown below. a) What is the initial boiling point of a solution that contains 50% toluene and 50%

Boiling point of a mixture of water and nitrobenzene is `99^(@)C`, the vapour pressure of water is. - YouTube
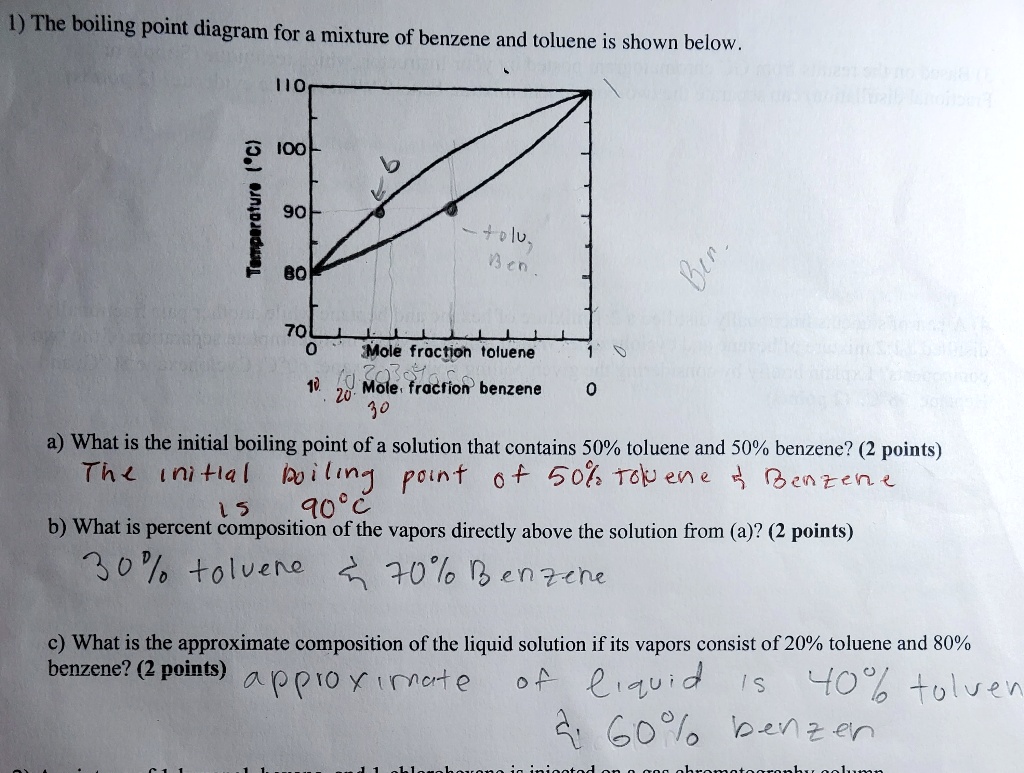
SOLVED: 1) The boiling point diagram for mixture of benzene and toluene is shown below 2 IOO 90 I 7 plu 13 < 70 'Mole froction ioluene 20' Mole, fraction benzene 10