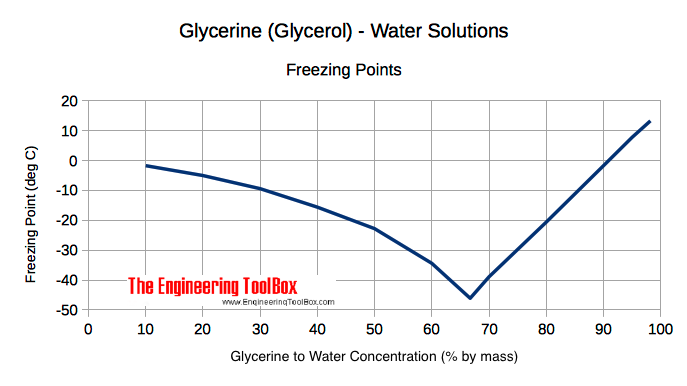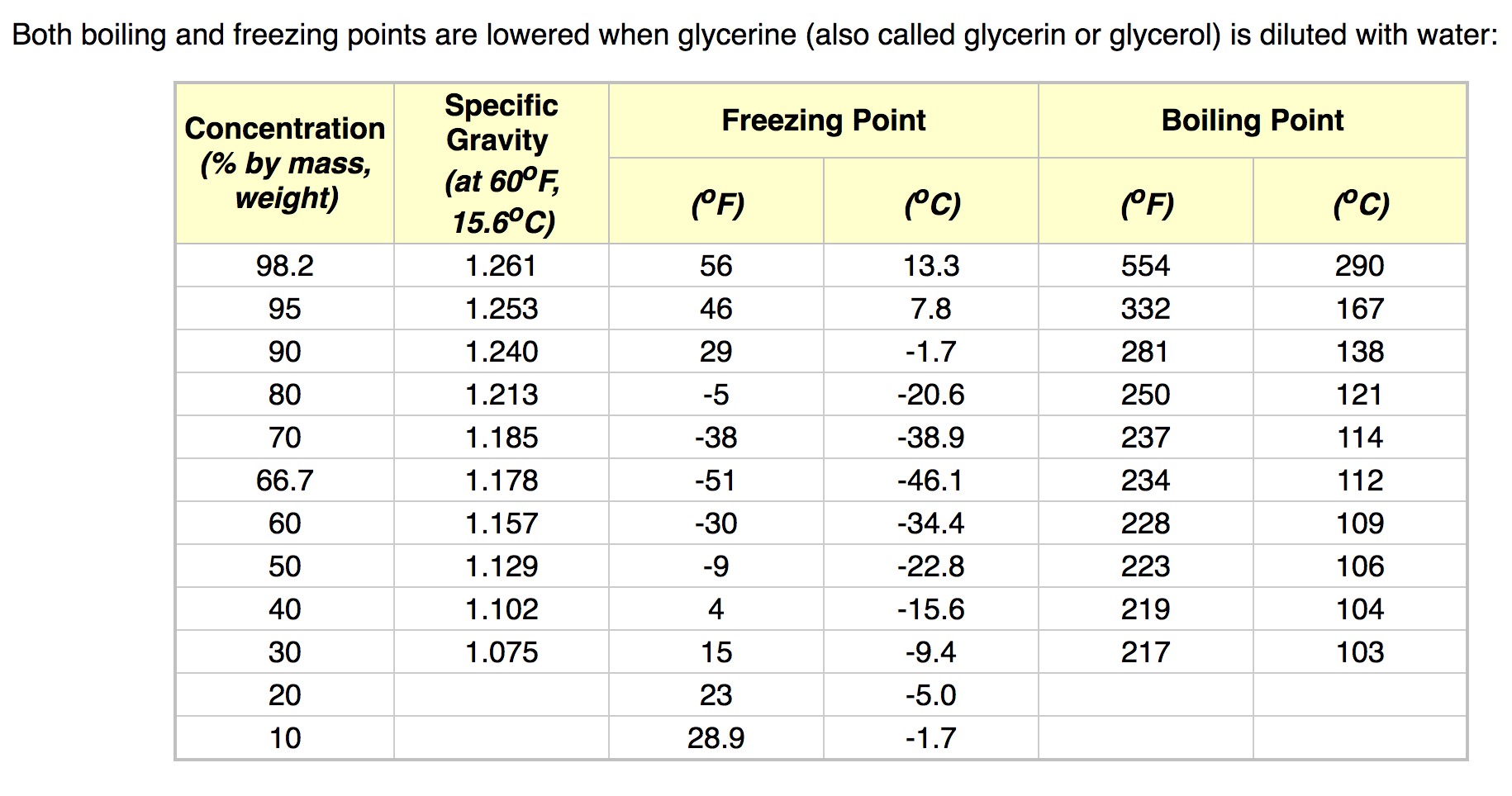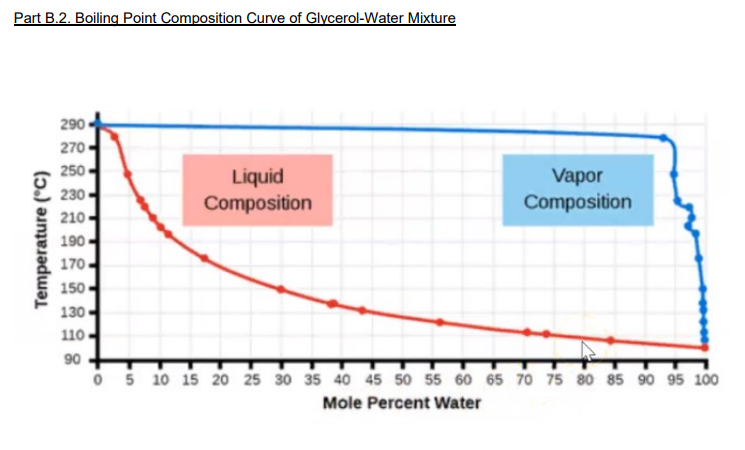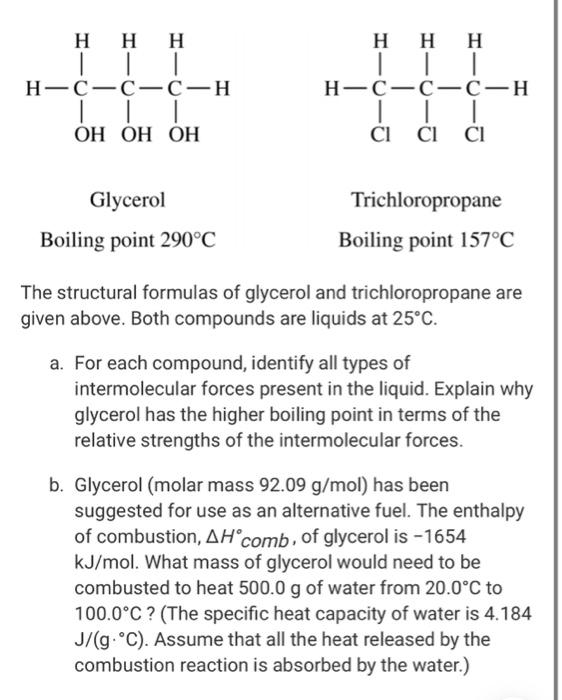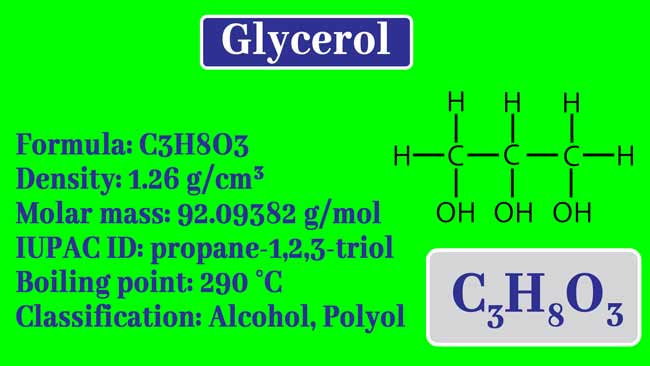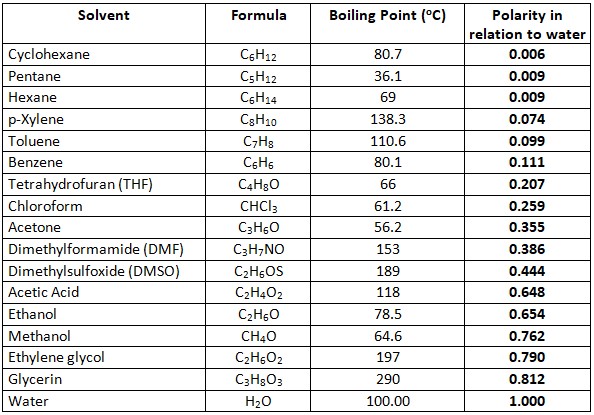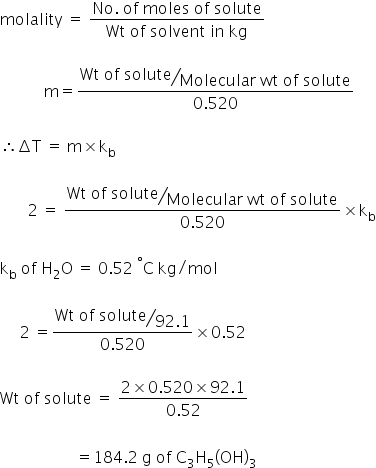
calculate the number of grams of glycerol c3h5 oh 3 mw 921g mol that must be dissolved in5 520 gm grams of water to raise the boiling point to 102000c 00nc4oii -Chemistry -

A solution of glycerol `(C-(3)H_(8)O_(3)` , molar mass = 92 g `mol^(-1)` iin water was prepared by - YouTube

Phase diagram of glycerol-water mixture with temperature (T/ • C) vs.... | Download Scientific Diagram

Glycerol decomposes at its boiling point (563 K) . Discuss a method which can used for its purification.

Vapor–Liquid Equilibrium of Water + Ethanol + Glycerol: Experimental Measurement and Modeling for Ethanol Dehydration by Extractive Distillation | Journal of Chemical & Engineering Data

Boiling points of the propylene glycol + glycerol system at 1 atmosphere pressure: 188.6-292 °C without and with added water or nicotine. - Abstract - Europe PMC