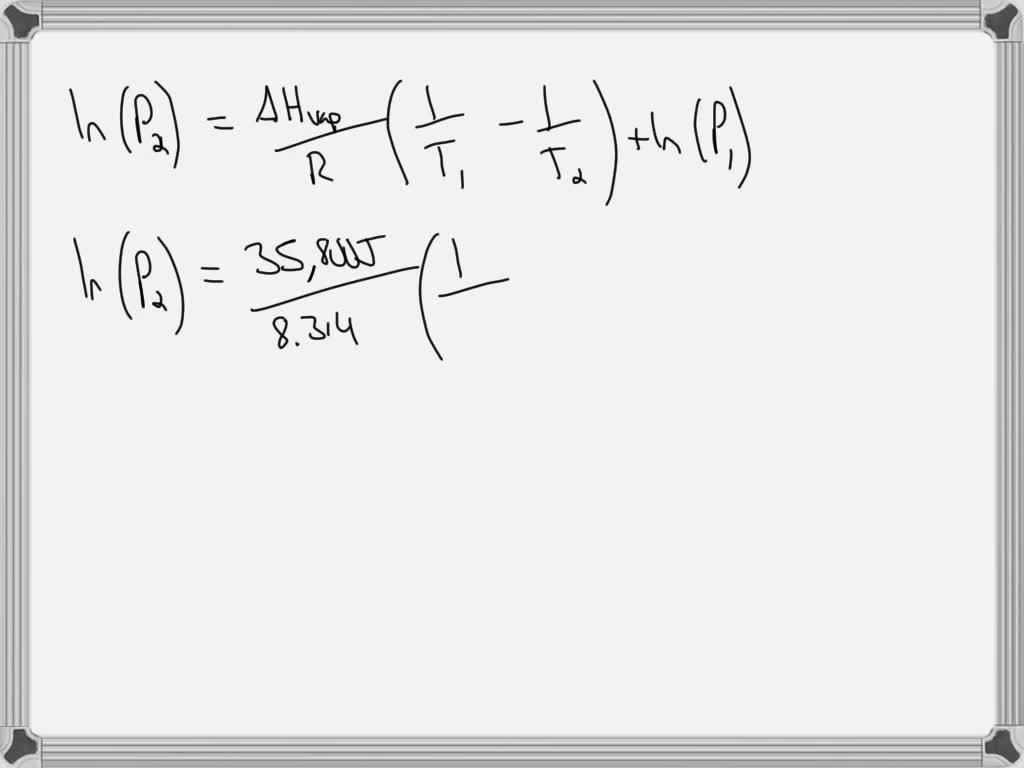Production of Isooctane from Isobutene: Energy Integration and Carbon Dioxide Abatement via Catalytic Distillation | Industrial & Engineering Chemistry Research

p-T diagram of iso-octane with possible fuel conditions for different... | Download Scientific Diagram

Vapor pressure measurements of ethanol–isooctane and 1-butanol–isooctane systems using a new ebulliometer - ScienceDirect

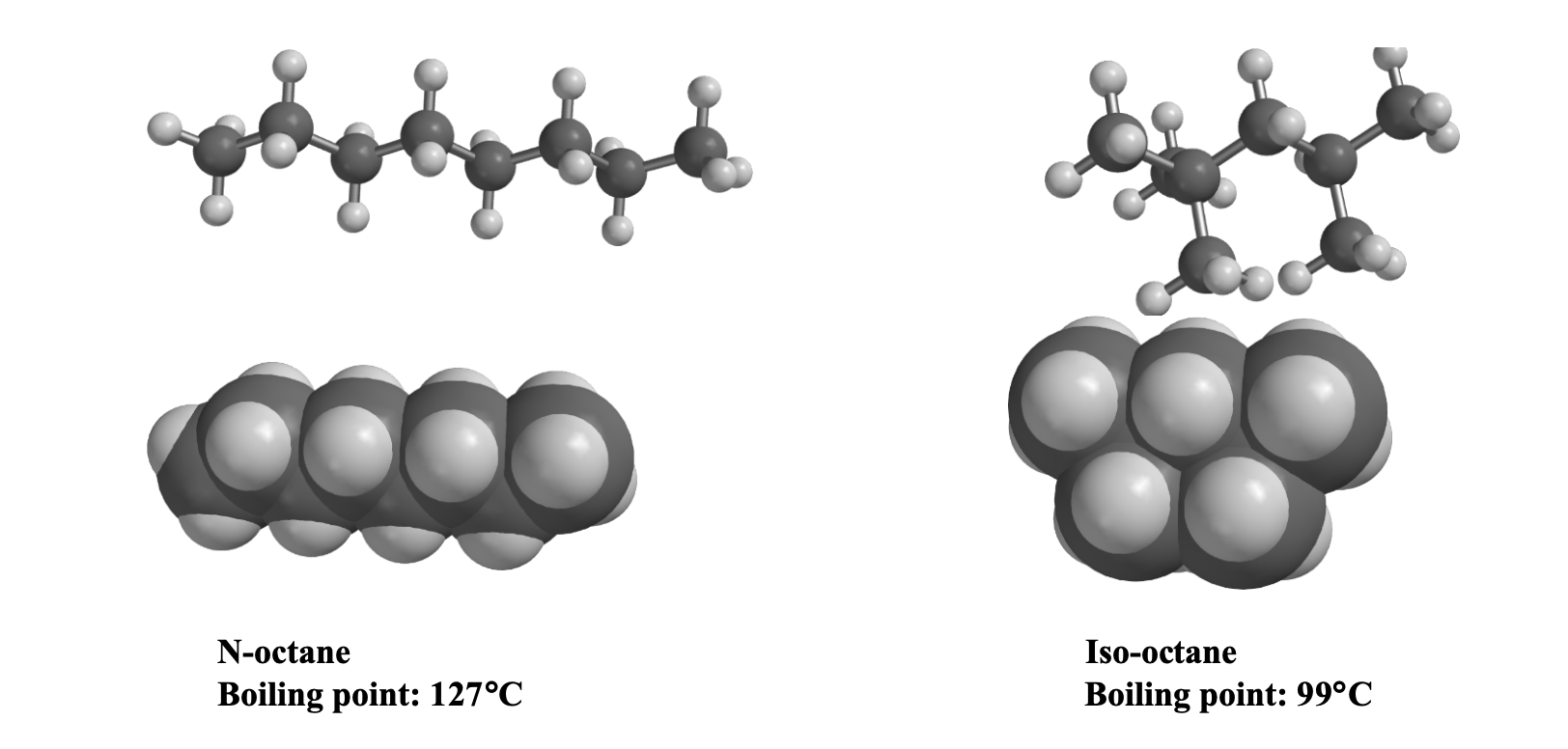
The role of molecule cluster on the azeotrope and boiling points of isooctane-ethanol blend - ScienceDirect

p-h-diagramm of iso-octane with an exemplary injection from 15 MPa fuel... | Download Scientific Diagram

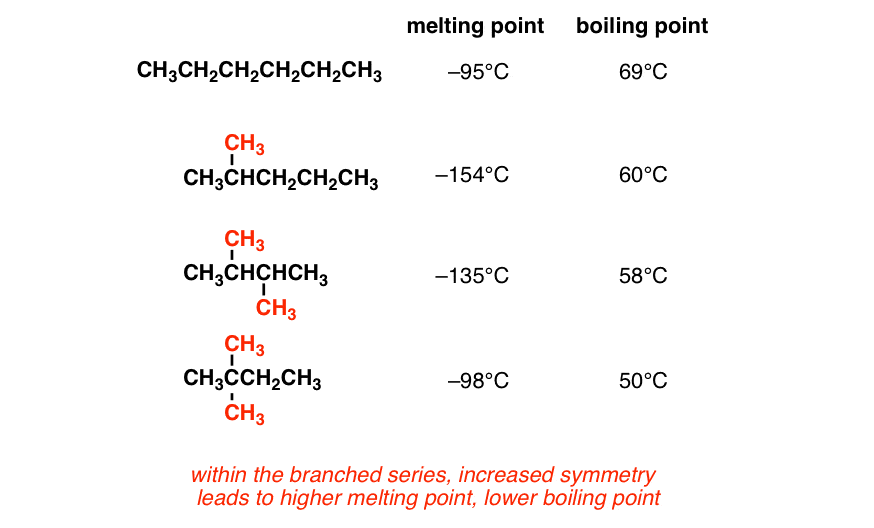
Volatility characteristics of the fuels used. ULG 78 is a mixture of... | Download Scientific Diagram
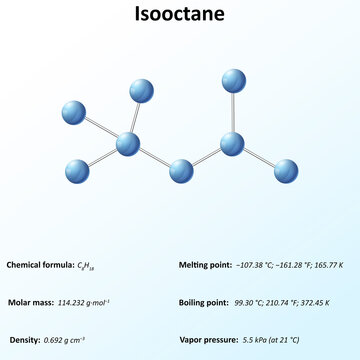
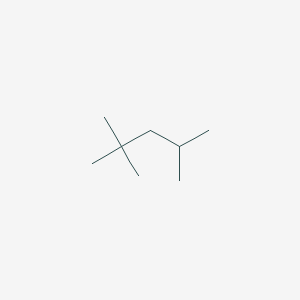
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18) Stock-Illustration | Adobe Stock
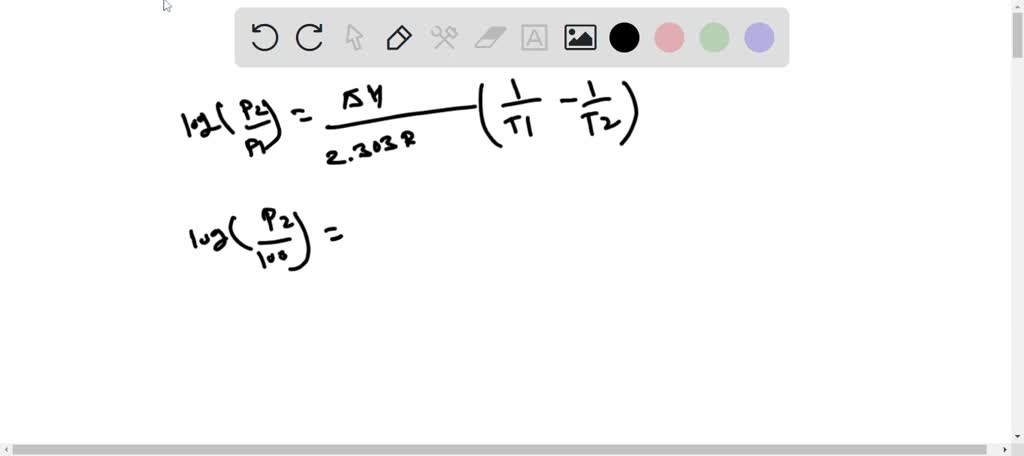
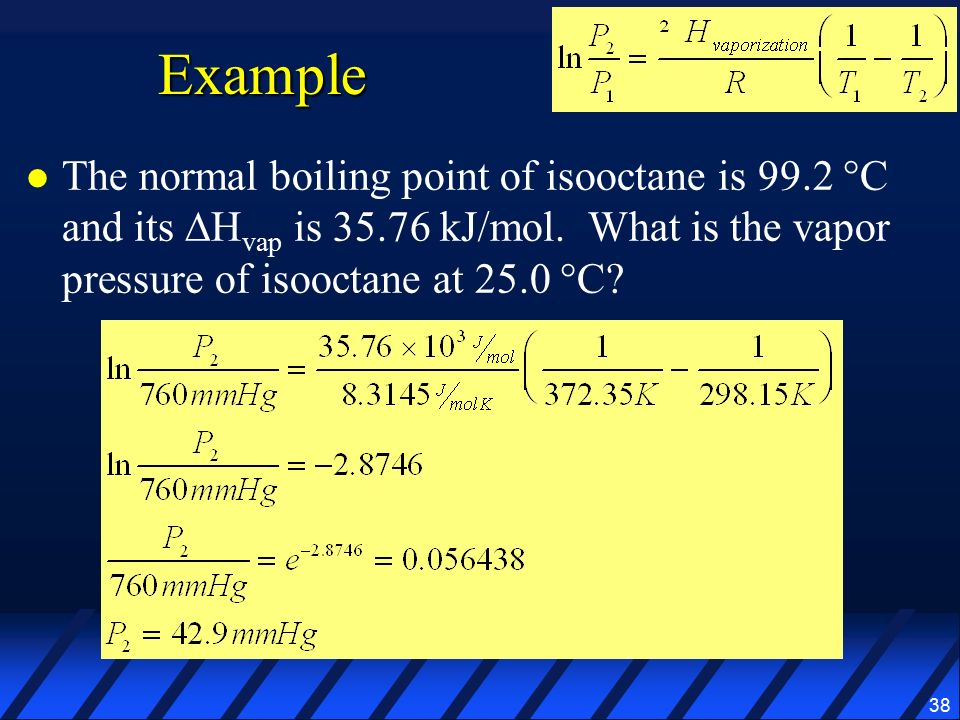
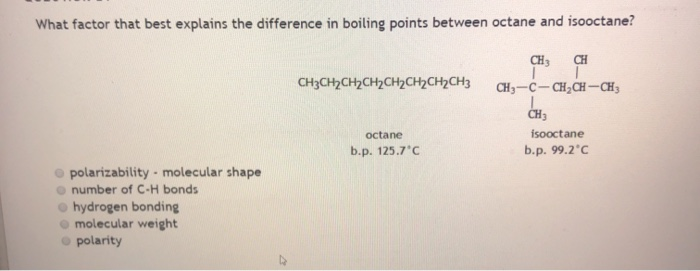
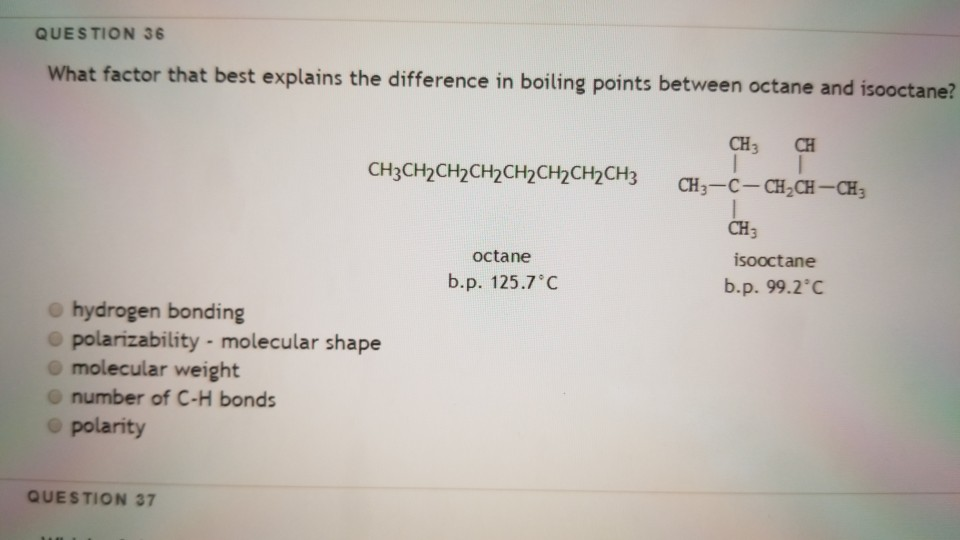
SOLVED: The normal boiling point of isooctane (C8H18) is 99°C and its enthalpy of vaporization is 35.8 kJ/mol. a) Calculate the vapor pressure of isooctane at 42°C, in mmHg. (You must show