EP0183110B1 - Azeotrope-like compositions of trichlorotrifluoroethane, ethanol, acetone, nitromethane and hexane - Google Patents
![SOLVED: The boiling point of hexane at 1 atm is 68.7oC. Estimate the vapor pressure of hexane at 30oC, using Trouton's rule. [26.2 kPa] The melting point of sodium is 370.6 K SOLVED: The boiling point of hexane at 1 atm is 68.7oC. Estimate the vapor pressure of hexane at 30oC, using Trouton's rule. [26.2 kPa] The melting point of sodium is 370.6 K](https://cdn.numerade.com/ask_previews/acec1919-118f-4f77-9d8a-b52a3d31110e_large.jpg)
SOLVED: The boiling point of hexane at 1 atm is 68.7oC. Estimate the vapor pressure of hexane at 30oC, using Trouton's rule. [26.2 kPa] The melting point of sodium is 370.6 K
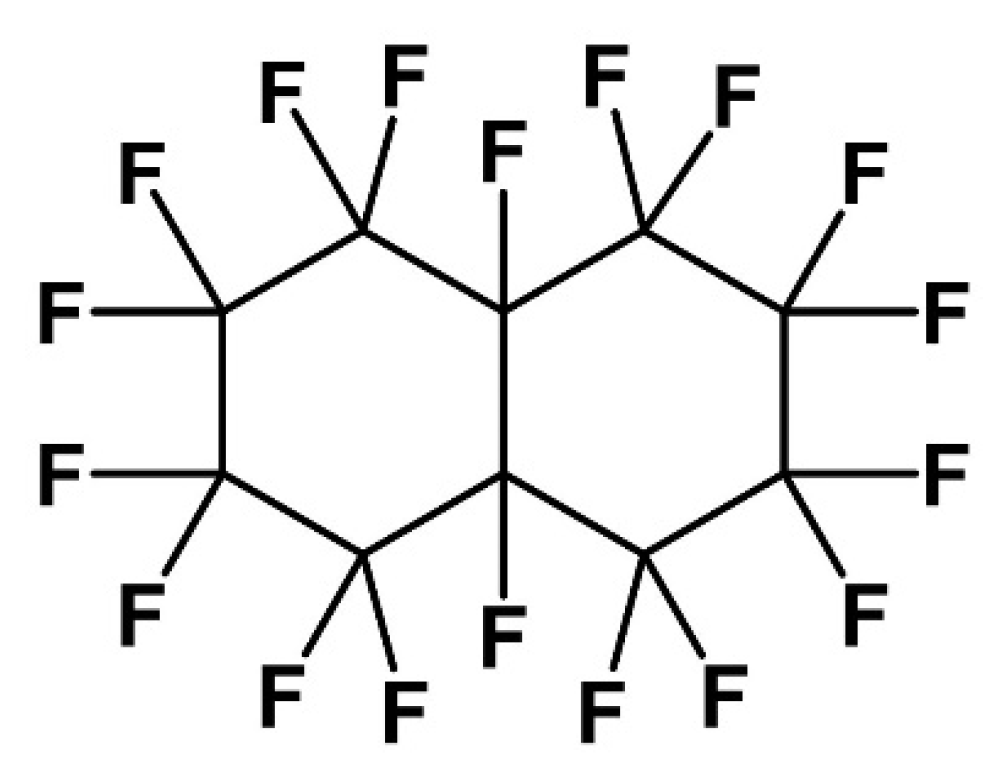
Liquids | Free Full-Text | Density and Dynamic Viscosity of Perfluorodecalin-Added n-Hexane Mixtures: Deciphering the Role of Fluorous Liquids

organic chemistry - Why does neopentane have a higher melting point than n-pentane? - Chemistry Stack Exchange

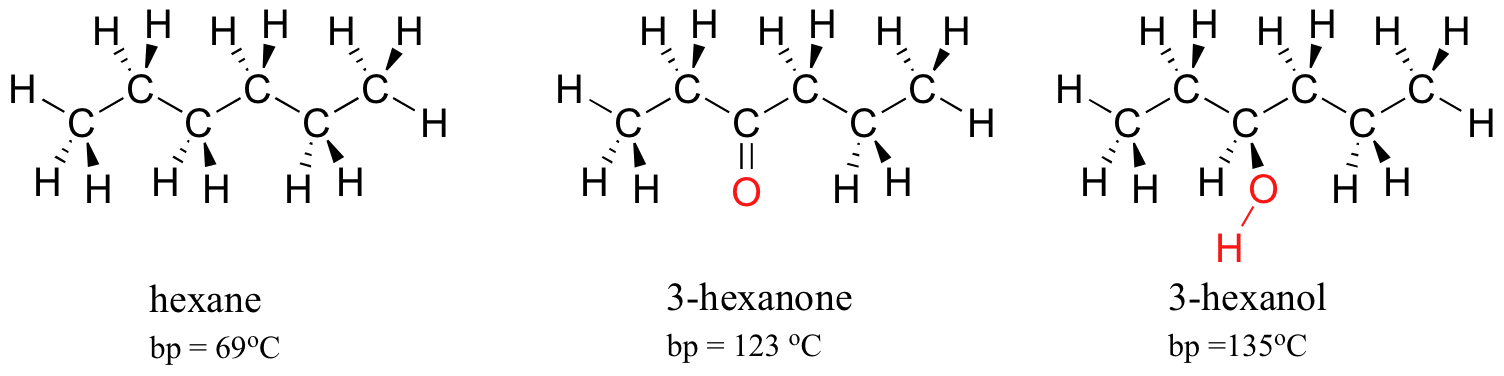
Explain which of the compounds hexane, 3-hexanon, and 1-hexanol is expected to have the highest boiling point and which one has the lowest boiling point. | Homework.Study.com

Boiling point of distillate as a function of weight fraction of hexane | Download Scientific Diagram

N-hexane vapor pressure curve and the chamber conditions at the start... | Download Scientific Diagram

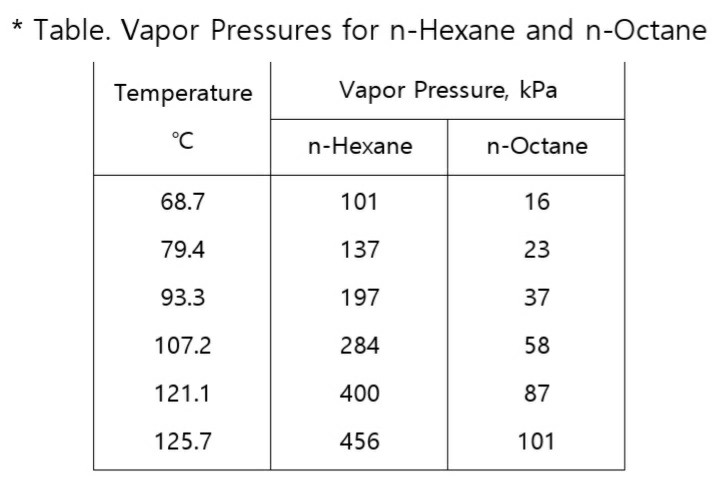
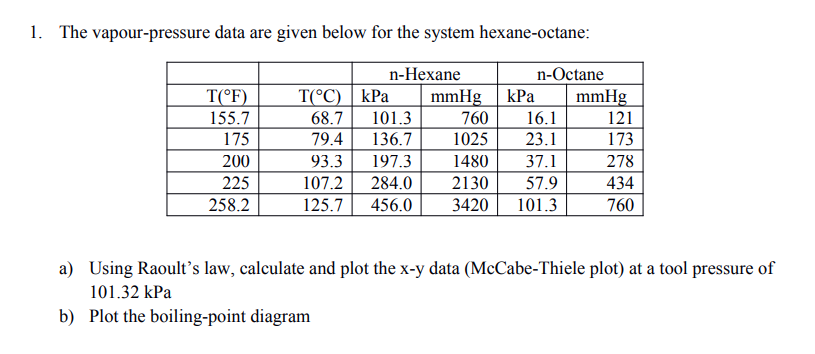
OneClass: For Problems 2 and 3, use the n-hexane, n-octane data from Problem 1. Number 1 answer is sh...

SOLVED: The normal boiling point of n-hexane Is 68.50 % At 20.00 %C, the vapour pressure of n-hexane is 0.1737 atm What is the vapour pressure of n- hexane when the temperature is

organic chemistry - Why do cyclic hydrocarbons have higher boiling points than their acyclic isomers? - Chemistry Stack Exchange