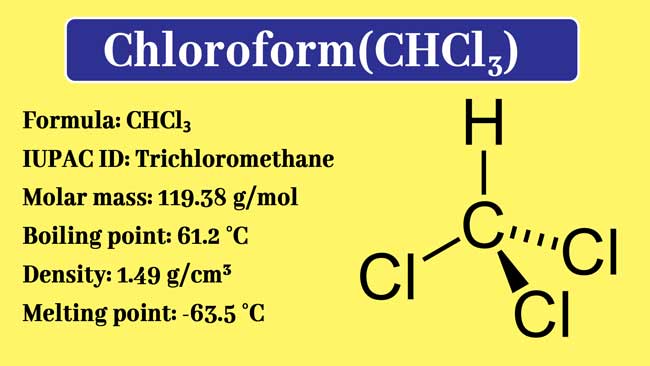
SOLVED: Chloroform (trichloromethane) , CHCls , has a normal boiling - point of 61.28C and an AHS enthalpy of 28.39 kJ/mol. of vaporization vap Estimate the entropy of vaporization Entropy of vaporization

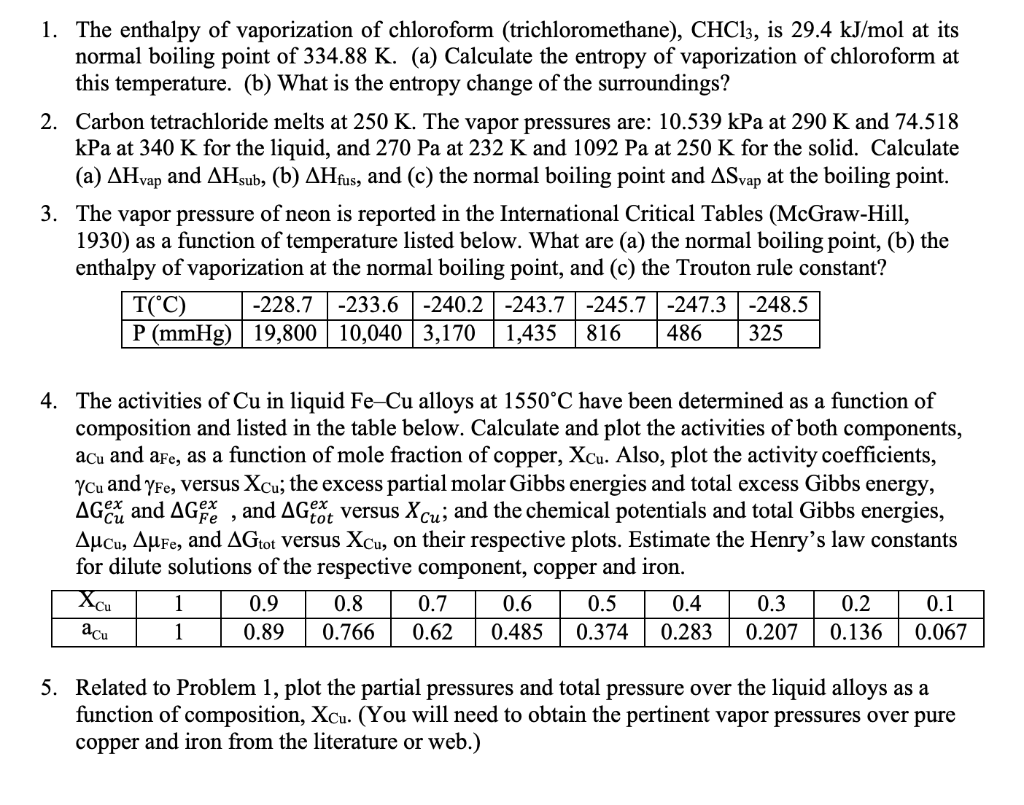
Write the correct decreasing order of boiling point for bromomethane , chloroform , dibromometha... - YouTube
What is the normal boiling point for chloroform?\n \n \n \n \n A. $40^\\circ C$B. $50^\\circ C$C. $60^\\circ C$D. $70^\\circ C$E. $80^\\circ C$

Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research

Why is the boiling point of trichlorofluoromethane lower than that of chloroform? - Chemistry Stack Exchange

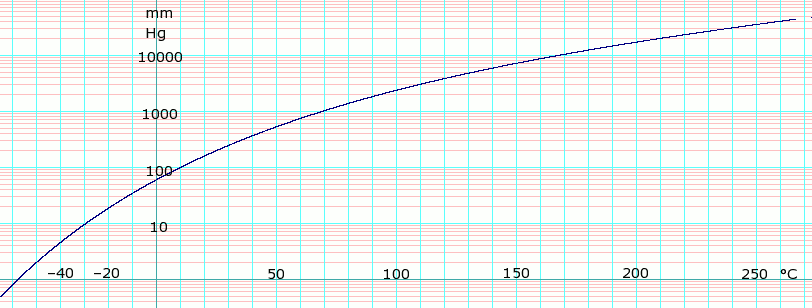
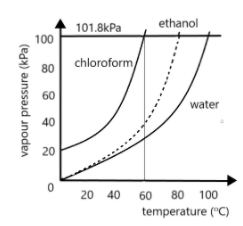
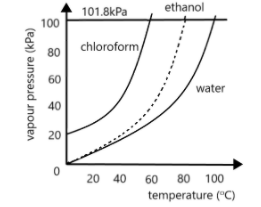
Use the figure below to determine the boiling point of -Chloroform at 80 kPa -Ethanol at 20kPa -Ethanol at - Brainly.com

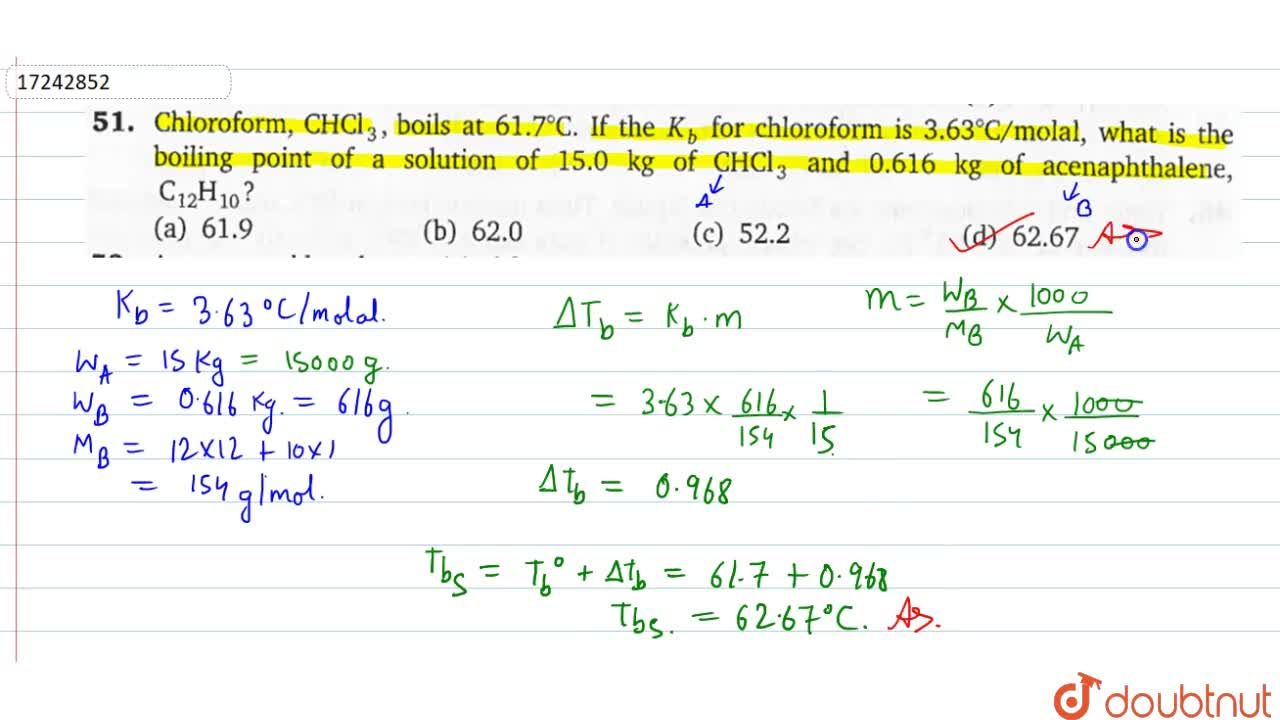
What would be the molar mass of a compound if 6.21 g of it dissolved in 24.0 g of chloroform form a solution that has a boiling point of 68.04^o C. The
What is the normal boiling point for chloroform?\n \n \n \n \n A. $40^\\circ C$B. $50^\\circ C$C. $60^\\circ C$D. $70^\\circ C$E. $80^\\circ C$
Trichloromethane/Chloroform, 10 l, tinplate, CAS No. 67-66-3 | Solvents for Synthesis | Solvents | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International
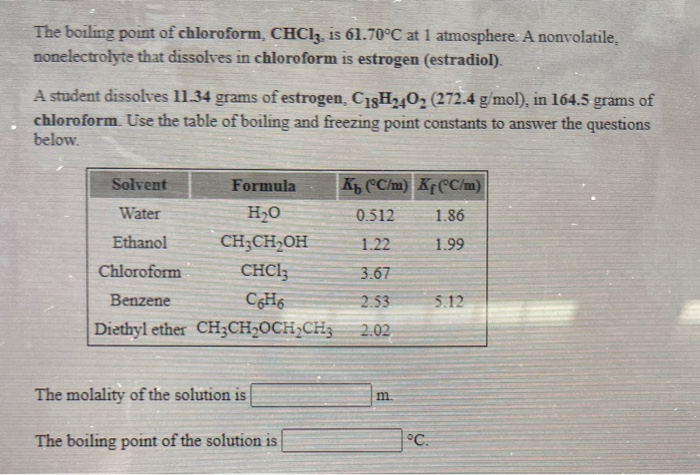
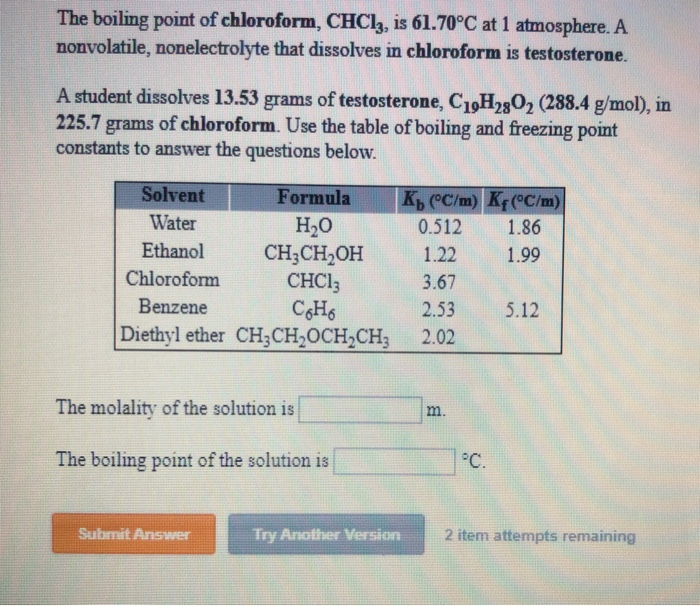
Boiling point of chloroform was raised by 0.323 K, when 0.5143 g of anthracene was dissolved in 35 g of chloroform. Molecular mass of anthracene isKb for CHCl 3=3.9 kg mol 1